Health & Wellness
Coastal Orthopedics Doctor a Leader for New Spinal Cord Study
Interventional pain management specialist Dr. Richard H. Bundschu (of Coastal Orthopedics Sports Medicine & Pain Management) is at the forefront of a new way physicians can help their patients manage chronic neck and arm pain.
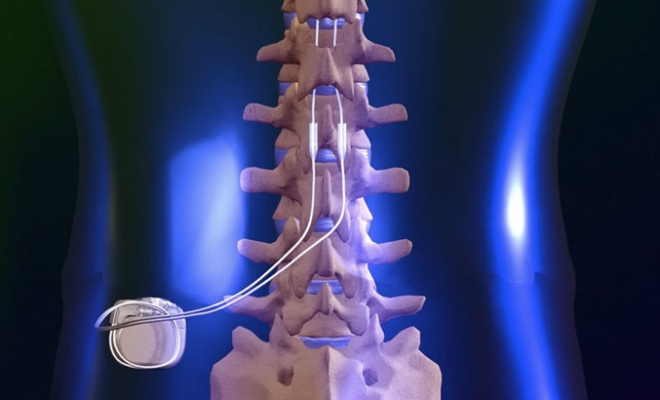
Dr. Bundschu is the principal investigator for the Nevro study — a landmark clinical trial to test the company’s spinal cord stimulators in treating ongoing neck and upper extremity body pain. Spinal cord stimulators have been used in the past to treat chronic pain in the lower back, torso and legs when pain medications, injections and physical therapy are ineffective.

“The whole idea is that this is not the first line of therapy,” Dr. Bundschu said. “It’s a very high-tech option for those people who have failed with other routes or treatments.”
Coastal Orthopedics was one of six locations nationwide selected to participate in the Nevro study, which began in June 2016. Bayshore Gardens resident Mike Daughtrey is participating in this trial and has experienced life-changing results.
After living with a chronic stiff neck for the past nine years, Daughtrey, 70, learned about the Nevro study while his wife, Donna, was being evaluated for a low back stimulator. He participated in a nine-day trial in September 2016 before receiving a permanent stimulator in October.
“I’m 85 to 90 percent better.” – Mike Daughtrey, pain patient
Within 36 hours, Daughtrey’s nightly headaches disappeared, and he was able to turn his head left and right without pain. Prior to receiving the stimulator, Daughtrey couldn’t look up at the stars, drive without turning his whole body or drink a can of soda without using a straw.
“It was truly like an angel reached down and touched my neck and made it better,” Daughtrey said. “If I had to give it a percentage, I would say I’m 85 to 90 percent better.”
Four months later, Daughtrey is once again able to work a part-time job. He can ride the motorcycle that had been parked for nearly a year because he didn’t feel safe. And he can tilt his head back to drink a can of soda.
“It’s the things you don’t think of,” Daughtrey said. “It doesn’t wake me up at night. It’s been a miracle. I don’t know anyone who wouldn’t want to have it done.”
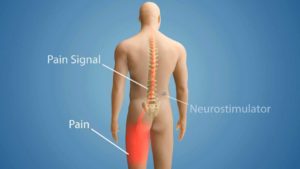
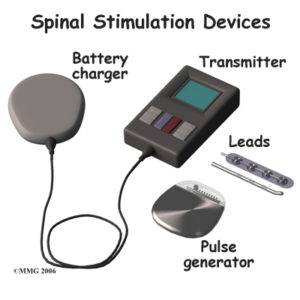
The neck stimulator resembles a pacemaker. A battery is installed above the hip and below the rib cage, in the area typically referred to as a “love handle,” while the electrodes are placed in the neck. An electric current is applied to the spinal nerves, which disrupts the flow of pain signals that travel to the brain. Patients are given a remote control where they can select a series of programs, ranging from one to five, depending on their level of pain.
“It’s electrical. It’s not chemical and the patient has control,” Dr. Bundschu said. “They are independent now.”
Enrollment in this trial is closed. Now, Dr. Bundschu will spend the remainder of the trial collecting data. He will meet with each of his patient’s every three months for the first year following implantation and possibly the second as well.
Dr. Bundschu worked with Nevro on two prior studies, including a low back study, which helped the company’s groundbreaking products earn U.S. Food and Drug Administration approval.
Last month, during a conference with 2,500 doctors, Dr. Bundschu learned the national study’s initial results. Although specific findings cannot be cited in an ongoing trial, the preliminary results showed a decrease in pain.
“I knew we had good results, but I didn’t know the results were that good nationwide,” Dr. Bundschu said. “The results of this neck and arm study are even better than the low back study.”

Source: Candice McElyea, ThreeSixOh PR




You must be logged in to post a comment Login